Atomic Structure and Periodic TrendsPage
16
16
d) All anions are larger than their parent atoms and all cations are smaller, compare Be2+(27 pm) and Be (112pm), I-(206pm) and I(133) – please note that ionic radius depends on coordination number of ion
e) Ionic radii generally decrease with increasing positive charge on the same ion (Tl+, 164pm > Tl3+, 88pm)
Slide 135
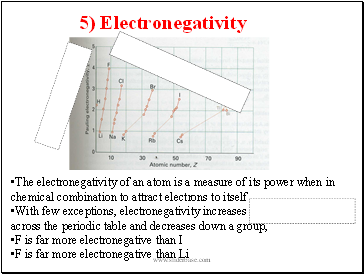
5) Electronegativity
The electronegativity of an atom is a measure of its power when in chemical combination to attract electrons to itself
With few exceptions, electronegativity increases across the periodic table and decreases down a group,
F is far more electronegative than I
F is far more electronegative than Li
Slide 136
Slide 137
Appendices
Slide 138
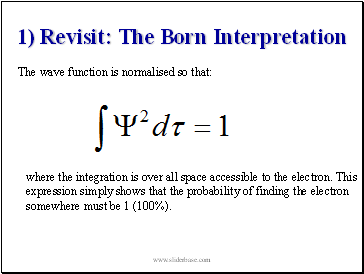
1) Revisit: The Born Interpretation
The wave function is normalised so that:
where the integration is over all space accessible to the electron. This expression simply shows that the probability of finding the electron somewhere must be 1 (100%).
Slide 139
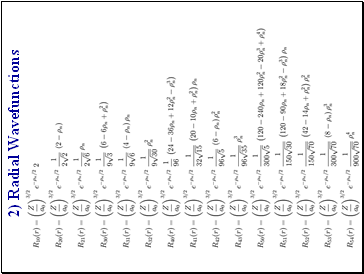
2) Radial Wavefunctions
Slide 140
r
z
y
x
dr
rdq
rsinqdf
df
f
q
q
r
rsinq
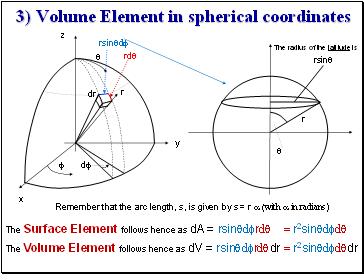
The radius of the latitude is
Remember that the arc length, s, is given by s = r a (with a in radians)
The Volume Element follows hence as dV = rsinqdfrdqdr = r2sinqdfdqdr
3) Volume Element in spherical coordinates
The Surface Element follows hence as dA = rsinqdfrdq = r2sinqdfdq
Slide 141
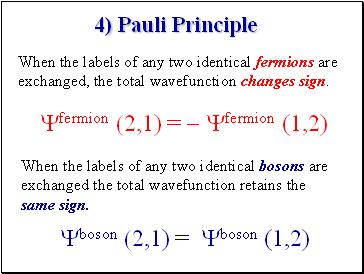
4) Pauli Principle
Yfermion (2,1) = - Yfermion (1,2)
Yboson (2,1) = Yboson (1,2)
When the labels of any two identical fermions are exchanged, the total wavefunction changes sign.
When the labels of any two identical bosons are exchanged the total wavefunction retains the same sign.
Slide 142
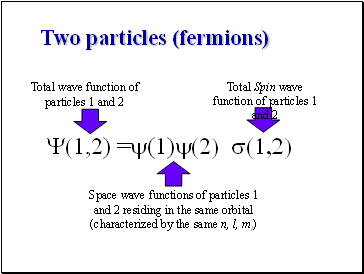
Two particles (fermions)
Y(1,2) =y(1)y(2) s(1,2)
Total wave function of particles 1 and 2
Space wave functions of particles 1 and 2 residing in the same orbital (characterized by the same n, l, ml)
Total Spin wave function of particles 1 and 2
Slide 143
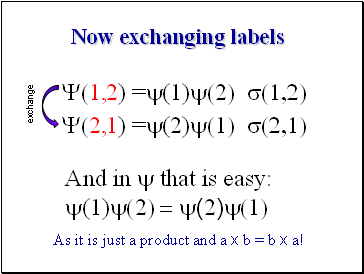
Now exchanging labels
As it is just a product and a x b = b x a!
Y(1,2) =y(1)y(2) s(1,2)
And in y that is easy:
y(1)y(2) = y(2)y(1)
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Solar Thermal Energy
- Solar Energy
- Newton’s Laws of Motion
- The Effects of Radiation on Living Things
- Newton’s law of universal gravitation
- Soil and Plant Nutrition
- Practical Applications of Solar Energy