Atomic Structure and Periodic TrendsPage
15
15
Ionization Potentials tend to for the successively heavier elements within a period as the number of protons in the nucleus increases and electrons are successively added to the same shell (Zeff increases at constant n).
However, some irregularities (penetration of p vs s and due to exchange energy contributions) occur.
Ionization Potentials tend to for the successively heavier elements in a group in the periodic table (as Zeff increases but n also increases and does so faster) .
Note transition metal and lanthanide contraction affect these trends.
The trends in second IE are similar but shifted by one atomic number:
The second IEs are than the first IE for that element.
Also, note particularly large increases as we start to take electrons from inner shells, e.g., the first, second and third ionisation energies of beryllium are: 899 kJ mol-1, 1756 kJ mol-1 and 14846 kJ mol-1.
Slide 130
More Periodic Trends
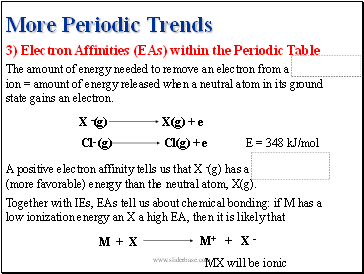
3) Electron Affinities (EAs) within the Periodic Table
X -(g)
X(g) + e
Cl- (g)
Cl(g) + e
E = 348 kJ/mol
The amount of energy needed to remove an electron from a negative ion = amount of energy released when a neutral atom in its ground state gains an electron.
Together with IEs, EAs tell us about chemical bonding: if M has a low ionization energy an X a high EA, then it is likely that
+ X -
M + X
M+
MX will be ionic
A positive electron affinity tells us that X -(g) has a lower (more favorable) energy than the neutral atom, X(g).
Slide 131
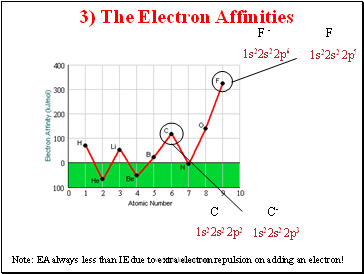
3) The Electron Affinities
1s22s2 2p5
1s22s2 2p6
F -
F
1s22s2 2p3
1s22s2 2p2
C
C-
Note: EA always less than IE due to extra electron repulsion on adding an electron!
Slide 132
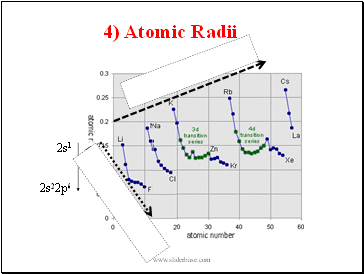
4) Atomic Radii
Decrease along period
Increase down group
Slide 133
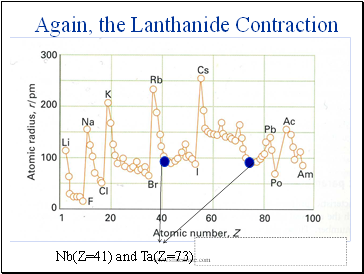
Again, the Lanthanide Contraction
Nb(Z=41) and Ta(Z=73) have identical atomic radii
Slide 134
4) Atomic Radii
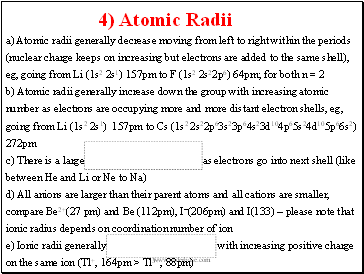
a) Atomic radii generally decrease moving from left to right within the periods (nuclear charge keeps on increasing but electrons are added to the same shell), eg, going from Li (1s2 2s1) 157pm to F (1s2 2s22p6) 64pm; for both n = 2
b) Atomic radii generally increase down the group with increasing atomic number as electrons are occupying more and more distant electron shells, eg, going from Li (1s2 2s1) 157pm to Cs (1s2 2s22p63s23p64s23d104p65s24d105p66s2)
272pm
c) There is a large increase as electrons go into next shell (like between He and Li or Ne to Na)
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Radioactivity and Nuclear Reactions
- Gravitation
- Direct heat utilization of geothermal energy
- Sound
- Solar Thermal Energy
- Solar Energy
- Heat-Energy on the Move